When and where did blue eyes appear? And why?
Reply to Davide Piffer
You’ve got your mother’s big blue eyes! Irving Berlin, 1913 (Wikicommons)
New eye and hair colors arose in Europe during the last ice age. They are more common in women than in men and seem to be associated with higher estrogen levels in the womb.
Archaeogeneticist Davide Piffer has reviewed the ancient DNA evidence on the origin of blue eyes among Europeans. The earliest evidence comes from an individual dated to around 35,000 years ago in the Crimea. This was during the last ice age, when Europeans were nomadic hunter-gatherers (Piffer, 2025).
But a lone individual may be just that. Davide concludes that blue eyes were confined to specific hunter-gatherer groups during glacial times. They then became much more common after the last ice age ended some 12,000 years ago.
Davide goes on to argue that blue eyes then became much less common when European hunter-gatherers were replaced by brown-eyed farmers from the Middle East. Afterwards, blue eyes quickly returned to their formerly high prevalence.
Here is my take on his argument:
Scarcity of aDNA from glacial times (>12,000 years ago)
Blue eyes are poorly attested from the ice age because so little DNA has survived from that time. Ice-age hunter-gatherers had a low population density, and many of their sites were destroyed by glaciation and glacial meltwaters. Post-glacial sites provide more DNA and more information on eye color. If we examine individuals who lived shortly after the ice age in Germany, Scandinavia, and the East Baltic, we find that half or a little over half were blue-eyed (Günther et al., 2018; Mittnik et al., 2018; Posth et al., 2023).
In sum, blue eyes were already common in northern Europe when the ice age ended and were probably common earlier.
Overestimation of population replacement by Anatolian farmers
Davide Piffer, like many archaeogeneticists, believes that the indigenous hunter-gatherers of Europe were considerably replaced by brown-eyed farmers from Anatolia. This belief leads him to argue that strong selection caused blue eyes to bounce back to their former prevalence.
Such a scenario requires rapid evolutionary change. Farming began to spread into northern Europe scarcely 6,000 years ago and into Finland and the East Baltic less than 4,000 years ago — and on a limited scale. The last date is already within the time of recorded history, and only a millennium before the Greek poet Homer mentioned blue eyes in his works.
There is no doubt that farmers did spread out of Anatolia and into Europe. But there is reason to doubt the claim that northern Europeans are 30 to 50% of Anatolian origin. This estimate assumes that any genetic change across the time boundary between hunter-gatherers and farmers is due to population replacement of the former by the latter. One such change is the loss of haplogroup U, which was common among Europeans before farming and is now found only among the Sami of Finland and the Mansi of Siberia, both of whom were hunter-gatherer-fishers until recently (Derbeneva et al., 2002). As expected, genomes from central and western Europe show a sharp break in the population frequency of haplogroup U at the time boundary between hunter-gatherers and farmers (Bramanti et al., 2009).
Yet things are not always what they seem. In Denmark, haplogroup U persisted at high frequencies long after the transition to farming — as late as the Early Iron Age (Melchior et al., 2010). In Latvia and Ukraine it persisted into the Neolithic (Jones et al., 2017). Is its disappearance better explained by natural selection? Haplogroup U shifts the body’s energy balance to heat production — a useful adaptation for hunter-gatherers when sleeping in makeshift shelters or pursuing game in all kinds of weather (Balloux et al., 2009). It would have been less useful for farmers, who slept in a warmer environment and could more easily plan their outdoor activities.
In addition to genetic changes due to differences in natural selection, there were also random changes in allele frequencies at the time boundary between hunter-gatherers and farmers. These are founder effects: only some hunter-gatherers adopted farming, and their allele frequencies would have differed somewhat from those who didn’t — again, for purely random reasons. Some of these stochastic changes create false similarities to the Anatolian genetic profile, thus inflating the estimated Anatolian ancestry of early farmers.
Because of these false positives, and because of convergent natural selection, we overestimate the Anatolian contribution to the North European gene pool, while underestimating the indigenous contribution.
Why blue eyes?
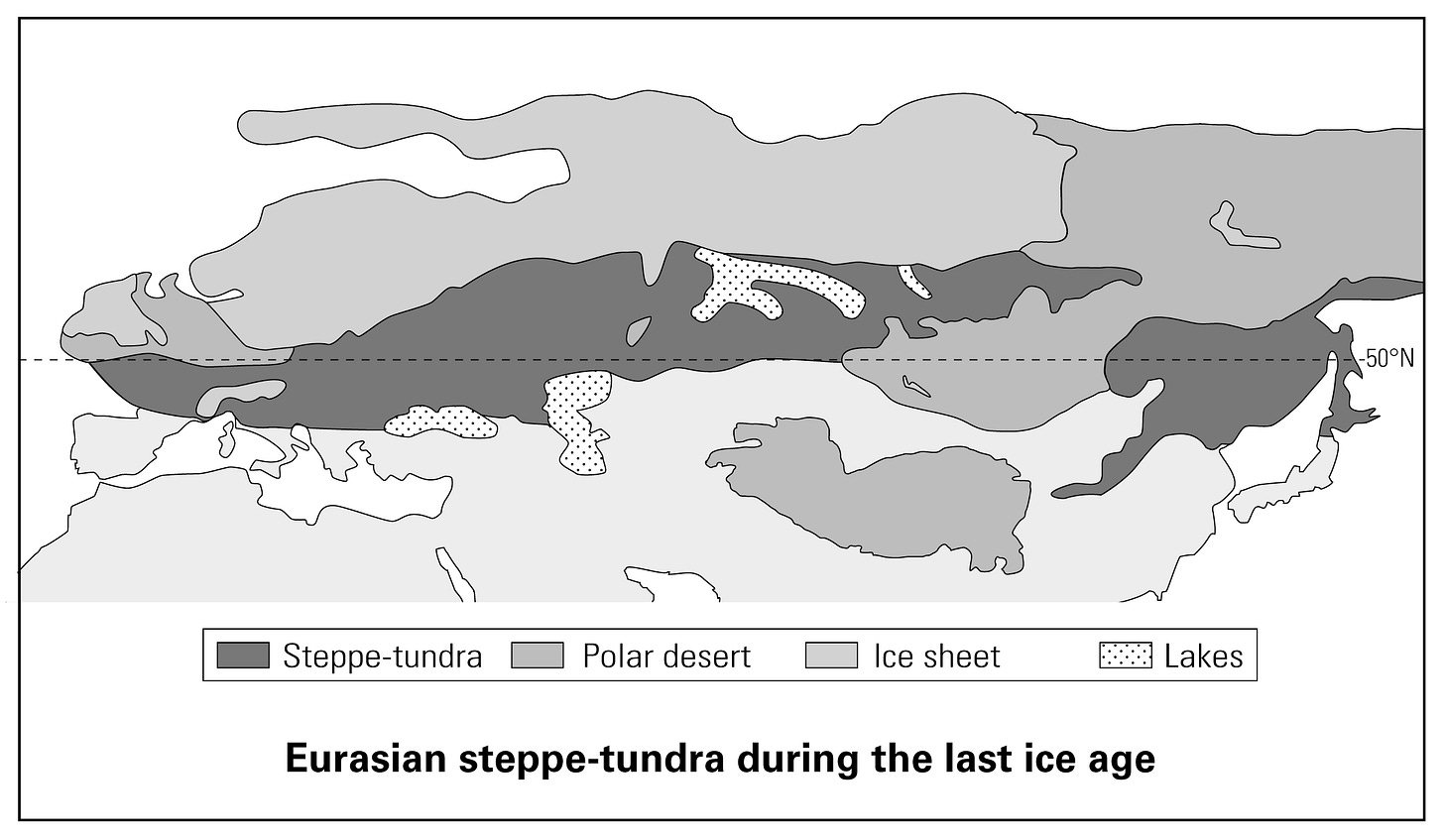
We know the answers to the “when” and the “where.” Blue eyes became common during the last ice age within a region encompassing Germany, Scandinavia, the East Baltic, and probably areas farther east.
At that time, Scandinavia and the Alps were under ice. Northern Europe was habitable only on the plains stretching from northern Germany eastwards. Before 12,000 years ago, these plains were steppe-tundra with wandering herds of reindeer and nomadic bands of hunter-gatherers. Actually, they were just hunters. There were few opportunities for gathering fruits, nuts, tubers, or edible greens. Food was almost entirely “meat on the hoof” (Hoffecker, 2002, pp. 8, 178, 193-194, 237).
But why would such an environment favor blue eyes? Davide offers four possible reasons:
Lower UV exposure requiring less melanin protection
Sexual selection for distinctive traits
Genetic drift in smaller northern populations
Need for lighter skin to maintain vitamin D synthesis where sunlight is weaker
Because their skin was white?
The first and last reasons imply a strong linkage between eye color and skin color. If selection causes skin to turn white, it should also cause eyes to turn blue. Actually, there is no such linkage. Pale skin often co-exists with dark eyes and dark hair, as noted by anthropologist Alice Brues:
If we were to take all the human beings in the world who have dark brown eyes and black or dark brown hair, we would not only have the vast majority of the human species, but would have a group which shows virtually the complete range of human skin color, from black to almost completely depigmented. (Brues, 1975)
A genome-wide association study of 192,986 Europeans has similarly concluded that eye color is not significantly associated with skin or hair color:
Although DNA variants within the MC1R gene are strongly associated with light skin and red hair color, no detectable association with eye color was found in our large GWAS, in line with previous albeit smaller-sized GWASs of more limited statistical power. Similarly, other DNA variants strongly associated with skin and hair color within genes, such as SILV, ASIP, and POMC, showed no statistically significant effect on eye color in this study, nor in previous studies. Moreover, we also identified 34 genetic loci that were significantly associated with eye color, but for which there is no report of significant association with hair and/or skin color. (Simcoe et al., 2021)
We might find a significant association by examining millions of Europeans, but it would be very weak and could hardly explain such a dramatic increase in the prevalence of blue eyes.
Because of genetic drift?
This explanation would be plausible if the change concerned the frequency of only one allele. To date, researchers have identified 124 alleles that together account for 53% of the diversity in European eye color (Simcoe et al., 2021). This is a massive increase in the number of alleles from the single allele that initially prevailed in ancestral Europeans and still does in most humans.
The word “diversity” is significant here. This is not simply the replacement of one color by another. It is a shift from one color to many. Eyes took on a broad range of hues, not only brown but also blue, gray, hazel, and green.
Only some sort of selection could have caused so many new colors to become so prevalent in so short a time — at most, the time since the arrival of modern humans in northern Europe some 35,000 years ago. Genetic drift seems even less likely if we consider that the diversification of eye color was paralleled by a diversification of hair color within the same region and timeframe — over 200 alleles for various permutations of black, brown, flaxen, blonde, and red (Morgan et al., 2018). In fact, the number may be even higher: a recent forensic model used 2,997 and 4,954 SNPs respectively to predict the existence of blonde and brown hair (Cabrejas-Olalla et al., 2025).
What are the chances of two color polymorphisms, one for the eyes and one for the hair, arising at different genes within the same limits of time and space? It also seems more than a matter of chance that two color polymorphisms would arise on or near the face — which is crucial for visual recognition.
Because of sexual selection?
The new colors are also brighter and purer than the original brown and black. A brighter color reflects more light. A purer color reflects light within a narrower slice of the visible spectrum. Bright, pure colors are typically created by selection of one sort or another to attract attention, be it from a pollinating bee or a potential mate.
All of this is consistent with sexual selection, which occurs when too many of one sex have to compete for too few of the other. What could have caused such an imbalance on the European steppe-tundra of the last ice age?
There were two relevant factors:
Low polygyny rate. Food was largely meat from herbivores, mostly reindeer. Because men were the hunters, they alone had to provision their wives and children with sufficient food. Only a very capable hunter could provide for two wives and their offspring. If single women were too numerous, only a few could pair up with a polygynous male.
High male death rate. Game animals had to be pursued over long distances: “hunter-gatherers in northern continental environments … must forage across large areas in order to secure highly dispersed and mobile prey” (Hoffecker, 2002, p. 8). Again, men were the hunters, and their risk of death was a function of hunting distance. The longer the distance, the higher their risk of death from drowning, starvation, exposure, lack of shelter, and other hunting-related accidents.
Together, these two factors led to a surplus of unmated females. Too many women had to compete for too few men. Women were thus under strong selection for colorful features that could attract male attention, including new hair and eye colors (Frost, 2006; Frost, 2022).
Today, hair and eye colors are most diverse within a region that roughly corresponds to the European steppe-tundra of the last ice age, if one allows for the cover of ice over Scandinavia and the Alps. Yet this steppe-tundra also extended into parts of North Asia. Why, then, do indigenous North Asians have only black hair and brown eyes?
The reason may be that the steppe-tundra was colder and drier in North Asia than in Europe, being farther north and further removed from the moderating influence of the Gulf Stream. The Asian steppe-tundra thus supported much smaller human populations, which frequently died out — especially during the glacial maximum. The effects of sexual selection were thus repeatedly reset to zero. Also, in a smaller population, selection has to wait longer for suitable alleles to appear through mutation.
Nonetheless, we do see frequent cases of light hair in certain indigenous groups of North Asia and even Arctic North America (Frost, 2009).
Steppe-tundra did exist in North Asia during the last ice age, but it was farther north, colder, and drier.
Women more often have the “new” hair and eye colors
If sexual selection of women created these colors, wouldn’t women be more prone to having them? This is indeed the case with natural hair color. Red hair is highly overrepresented among women, followed by blond hair and light brown hair, whereas black hair is three to five times underrepresented (Hysi et al., 2018; Shekar et al., 2008).
For eye color, green and hazel are overrepresented among women while brown and blue are underrepresented (Frost, Kleisner, & Flegr, 2017). Why are blue eyes less frequent among women? Isn’t it a “new” color?
It looks like sexual selection favored not only bright and pure colors but also rare ones. There seems to be a novelty effect: the less frequent an eye or hair color, the more it attracts attention. Thus, as new colors arose through mutation, they benefitted from their novelty and increased in frequency at the expense of more common colors. The colors green and hazel appear to have arisen later as a novel form of blue eye color; hence, a greater proportion of blue eyes are expressed in women as green or hazel.
This novelty effect is described in Darwin’s discussion of sexual selection: “It would even appear that mere novelty, or slight changes for the sake of change, have sometimes acted on female birds as a charm, like changes of fashion with us” (Darwin, 1936[1888], p. 813). Like other aspects of visual merchandising, it is most important in saturated markets that offer too many interesting choices among products of equal quality (Lea-Greenwood, 1998).
This rare-color advantage has been reported by several studies.
An American research team showed pictures of attractive women to male participants and asked them to choose the one they most wanted to marry. There were three series of pictures: the first had equal numbers of brunettes and blondes; the second had one brunette for every five blondes; and the third had one brunette for every eleven blondes. The scarcer the brunettes were in a series, the more often they were chosen (Thelen, 1983).
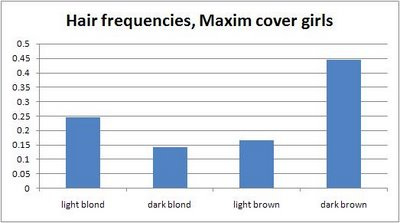
Maxim cover girls are likelier to be light blonde or dark brown than the more common dark blond or light brown of real life (Anon, 2008a).
Viennese women tend to change their hair to a less common color (Schweder, 1994).
In Brazil and the United Kingdom, modeling agencies prefer women with less frequent eye colors (Forti & Young, 2016).
On American TV, women are four and a half times likelier than men to have red or auburn hair and five times likelier to have blonde hair (Davis, 1990).
On Turkish TV, women more often have red or blonde hair (Ikizler, 2007, p. 39).
Blonde Playboy playmates have become more frequent since the mid-1960s, increasing from a third to over half the total (Anon, 2008b). This trend may be driven by the declining prevalence of blondes in the American population.
A novelty effect can also be seen in the way a preference for one paint color for home interiors would rise until it became satiated and then give way to preference for another (Stansfield & Whitfield, 2005).
However, no such rare-color advantage was found in a replication of the original American study. It should be noted that the replication differed from the original in two ways: participants were recruited online and made their choices privately on their home computers, with no control over whatever female images they had viewed previously or might still be viewing within sight of their computer screen (Janif et al., 2015).
Less frequent hair colors seem to be chosen more often for Maxim cover girls (Anon, 2008a).
Estrogen facilitates fetal development of European hair and eye colors
This sex difference in hair and eye colors may have a hormonal cause. Specifically, estrogen seems to favor the expression of non-black hair and non-brown eyes during fetal development.
An estrogenic effect is especially likely for red hair, which is the hair color that differs the most in prevalence between men and women. According to a survey of 7,000 Czech participants, male redheads are as healthy as other men, doing better on average in three categories and worse in three. Female redheads, however, do worse on average than other women in ten categories and better in only three. They are especially prone to four types of cancer: colorectal, cervical, uterine, and ovarian — three of which are estrogen-dependent. Being both female and red-haired seems to maximize the risk of developing an estrogen-dependent disease, probably because these risk factors are both associated with higher estrogen levels in a developing fetus (Frost et al., 2017).
A higher degree of fetal estrogenization may explain why several studies have found an association between blue eyes and certain feminine traits. [Note: although blue eyes are actually less frequent in women than in men, these studies used a broad definition of “blue” that included green and hazel, which are more frequent in women].
Blue-eyed boys tend to be shy. This is the “little boy blue” effect. A study of preschoolers found more social wariness in blue-eyed boys than in brown-eyed boys. The difference was greatest at the extremes of wariness. Among the very inhibited boys, 13 out of 14 were blue-eyed. Among the very uninhibited, only 4 out of 10 were. There was no such relationship among the girls, whose eyes were blue in 5 out of 9 among the very inhibited and in 6 out of 11 among the very uninhibited (Coplan et al., 1988).
Blue-eyed women tend to have narrower shoulders and higher hip-to-waist ratios. A Latvian study found small but significant correlations between female eye color and certain sexually dimorphic features. Shoulders were narrower and hip-to-waist ratios higher in blue-eyed women than in brown-eyed women (Kažoka & Vētra, 2011).
Blue-eyed men tend to have more feminine faces. This was an unintended finding of two Czech studies whose participants were asked to rate male and female facial photos. Initially, the brown-eyed male faces were rated as more dominant than the blue-eyed male faces. When, as a control, the brown-eyed faces were photoshopped to make them blue-eyed, they were still rated as more dominant. On careful examination, the originally brown-eyed faces were found to be more masculine with broader and more massive chins, broader mouths, larger noses, larger eyebrows, and closer-set eyes. The originally blue-eyed faces had smaller and sharper chins, narrower mouths, smaller noses, and greater distance between the eyes.
Blue eyes were associated with a more feminine facial shape only when the faces were male, just as blue eyes were associated with shyness only in boys. Perhaps natural selection has ensured that female fetuses will always receive enough estrogen for their physical and behavioral development. Male fetuses may be exposed to a range of estrogen levels with more variable effects (Kleisner et al., 2010; Kleisner et al., 2013).
Were brown eyes associated with a different facial shape in this study because some of the brown-eyed men were partly Jewish or Roma and had a more Mediterranean appearance? In that case, facial shape would have been more variable in the brown-eyed men. It was not. This explanation also fails to explain the effect of gender: why were blue eyes associated with facial feminization in men but not in women?
The sex hormones seem to affect hair color not only in the womb but also at puberty. Alleles for darker hair color are associated with earlier puberty in men, and a similar but weaker association exists in women. This looks like a testosterone effect (Hollis et al., 2020). There may thus have been some counter-selection acting on men to resist changes to their physical appearance due to spill-over from sexual selection acting on women.
Eventually, sexual selection would have produced more contrasting sex differences in hair and eye color. It didn’t because it occurred over a relatively brief period, essentially between the entry of modern humans into northern Europe some 35,000 years ago and the end of the ice age some 12,000 years ago. Briefer still was the period of intense sexual selection — the glacial maximum between 20,000 and 15,000 years ago.
Averaged faces of blue-eyed men (left) and brown-eyed men (right), Czech population (Kleisner et al., 2010).
White skin
What about the strange albino-like skin of Europeans? Could it also be due to sexual selection?
The counter-argument is that skin became white at high European latitudes to maintain sufficient synthesis of vitamin D. But why, then, did it fail to whiten to the same degree among indigenous peoples at the same latitudes in Asia and North America? Even in Europe, modern humans remained brown-skinned long after they arrived in the continent. Three research teams have estimated that white skin arose somewhere in Europe no earlier than 19,000 years ago (Canfield et al., 2014; Beleza et al., 2013; Norton and Hammer, 2007). In western Europe, Europeans remained brown-skinned until almost the dawn of history, as shown by DNA dated to 8,000 years ago from Luxembourg, 7,000 years ago from Spain, and as late as 5,000-4,000 years ago for some people in England (Brace et al., 2019; Lazaridis et al., 2014; Olalde et al., 2014).
One could argue that it was the change from hunting to farming that caused skin color to turn white. With Europeans consuming less meat, their bodies required more vitamin D. There is in fact evidence that meat contains a cofactor that independently reduces the risk of rickets (Dunnigan et al., 2005). But we still have to explain why some European populations were white-skinned long before farming. Two ancient DNA studies have shown a high prevalence of white skin in Scandinavians 9,500 to 6,000 years ago and in East Baltic peoples 7,460 to 5,360 years ago (Günther et al., 2018; Mittnik et al., 2018).
So what made Europeans white? Probably the same sexual selection that made their hair and eyes diversely colored. Unlike their hair and eyes, however, their skin did not acquire a variety of colors. It just became pale. This may be because sexual selection for light skin was guided by a pre-existing sexual dimorphism; i.e., women are lighter-colored than men across all human populations (Edwards & Duntley, 1939; Edwards & Duntley, 1949; Edwards et al., 1941; Frost, 2023; Manning et al., 2004). Lighter-skinned women are thus seen as more feminine in traditional cultures and preferred as mates (van den Berghe & Frost, 1986). When sexual selection is sufficiently strong, it should drain the gene pool of alleles for dark skin.
Ultimately, women have a lighter skin for the same reason they have a smaller nose and chin, a smoother, more pliable skin, and a higher pitch of voice. These are visual, tactile, and auditory cues that originally identified the human infant to an adult observer, who would respond by being less aggressive and more willing to provide care and nurturance. By mimicking those cues, women could strengthen the pair-bond and better ensure male provisioning (Frost, 2010, pp. 134-135; Frost, 2011; Frost, 2025; Lorenz, 1971, pp. 154-164).
Is the European face primarily a woman’s face?
When modern humans arrived in northern Europe some 35,000 years ago, they looked much like other modern humans elsewhere. They then became more and more different-looking, not through selection by the natural environment but through selection by the limited supply of men. On the steppe-tundra of the last ice age, the high male death rate and the high cost of polygyny for men created a continual surplus of unmated women. The resulting increase in sexual selection favored those women who could better catch male attention.
Sexual selection probably peaked during the glacial maximum some 20,000 to 15,000 years ago. Although women were the ones being selected, the effects spilled over onto men due to the weak sex-linkage of skin, hair, and eye color. Both sexes thus acquired the white skin and bright hair and eye colors that initially arose among women.
This sexual selection ended with the end of the last ice age. For reasons unrelated to their physical appearance, these pale humans would spread across Europe during post-glacial times, pushing west and south out of their original territory and eventually replacing their darker cousins throughout the continent. Thus, on the eve of recorded history, all Europeans now possessed a unique look that would later define them, as if they were a cast of actors being hastily made up and rushed onto stage moments before curtain time.
References
Anon. (2008a). Maxim’s audience prefers brunettes; distribution is bimodal. Gene Expression, July 6. http://www.gnxp.com/blog/2008/07/maxims-audience-prefers-brunettes.php
Anon. (2008b). Bygone brunette beauty: Fashion in hair color. Gene Expression, June 29. http://www.gnxp.com/blog/2008/06/bygone-brunette-beauty-fashion-in-hair.php
Balloux F., Handley, L.J., Jombart, T., Liu, H., & Manica, A. (2009). Climate shaped the worldwide distribution of human mitochondrial DNA sequence variation. Proceedings of the Royal Society B. Biological Sciences, 276, 3447-3455. https://doi.org/10.1098/rspb.2009.0752
Beleza, S., Santos, A.M., McEvoy, B., Alves, I., Martinho, C., Cameron, E., et al. (2013). The timing of pigmentation lightening in Europeans. Molecular Biology and Evolution, 30(1), 24-35. https://doi.org/10.1093/molbev/mss207
Brace, S., Diekmann, Y., Booth, T.J., Faltyskova, Z., Rohland, N., Mallick, S., et al. (2019). Ancient genomes indicate population replacement in Early Neolithic Britain. Nature Ecology & Evolution, 3(5), 765-771. https://doi.org/10.1038/s41559-019-0871-9
Bramanti, B., Thomas, M.G., Haak, W., Unterlaender, M., Jores, P., Tambets, K., Antanaitis-Jacobs, I., Haidle, M.N., Jankauskas, R., Kind, C.J., et al. (2009). Genetic discontinuity between local hunter-gatherers and Central Europe’s first farmers. Science, 326, 137-140. https://doi.org/10.1126/science.1176869
Brues, A.M. (1975). Rethinking human pigmentation. American Journal of Physical Anthropology, 43(3), 387-391. https://doi.org/10.1002/ajpa.1330430320
Cabrejas-Olalla, A., Jørgensen, F. G., Cheng, J. Y., Kjærgaard, P. C., Schierup, M. H., Mailund, T., & Athanasiadis, G. (2025). Genetic predictions of eye and hair colour in the Danish population. Forensic Science International: Genetics, 78, 103267. https://doi.org/10.1016/j.fsigen.2025.103267
Canfield, V.A., Berg, A., Peckins, S., Wentzel, S.M., Ang, K.C., Oppenheimer, S., & Cheng, K.C. (2014). Molecular phylogeography of a human autosomal skin color locus under natural selection. G3, 3(11), 2059-2067. https://doi.org/10.1534/g3.113.007484
Coplan, R., Coleman, B., & Rubin, K. (1998). Shyness and little boy blue: Iris pigmentation, gender, and social wariness in preschoolers. Developmental Psychobiology, 32(1), 37-44. https://doi.org/10.1002/(SICI)1098-2302(199801)32:1<37::AID-DEV4>3.0.CO;2-U
Darwin, C. (1936 [1888]). The Descent of Man and Selection in relation to Sex. reprint of 2nd edition, The Modern Library, New York: Random House.
Davis, M. D. (1990). Portrayals of women in prime-time network television: Some demographic characteristics. Sex Roles, 23(5-6), 325-332. http://psycnet.apa.org/doi/10.1007/BF00290052
Derbeneva, O.A., Starikovskaya, E.B., Wallace, D.C., & Sukernik, R.I, (2002). Traces of early Eurasians in the Mansi of Northwest Siberia revealed by mitochondrial DNA analysis. American Journal of Human Genetics, 70, 1009-1014. https://doi.org/10.1086/339524
Dunnigan, M.G., Henderson, J.B., Hole, D.J., Mawer, E.B., & Berry, J.L. (2005). Meat consumption reduces the risk of nutritional rickets and osteomalacia. British Journal of Nutrition, 94, 983–991. https://doi.org/10.1079/BJN20051558 .
Edwards, E.A., & Duntley, S.Q. (1939). The pigments and color of living human skin. American Journal of Anatomy, 65(1), 1-33. https://doi.org/10.1002/aja.1000650102
Edwards, E.A., & Duntley, S.Q. (1949). Cutaneous vascular changes in women in reference to the menstrual cycle and ovariectomy. American Journal of Obstetrics & Gynecology, 57(3), 501-509. https://doi.org/10.1016/0002-9378(49)90235-5
Edwards, E.A., Hamilton, J.B., Duntley, S.Q., & Hubert, G. (1941). Cutaneous vascular and pigmentary changes in castrate and eunuchoid men. Endocrinology, 28(1), 119-128. https://doi.org/10.1210/endo-28-1-119
Forti, I.R.N., & Young, R.J. (2016). Human commercial models’ eye colour shows negative frequency-dependent selection. PLoS One, 11(12) e0168458. https://doi.org/10.1371/journal.pone.0168458
Frost, P. (2006). European hair and eye color - A case of frequency-dependent sexual selection? Evolution and Human Behavior, 27(2), 85-103. https://doi.org/10.1016/j.evolhumbehav.2005.07.002
Frost, P. (2009). Blond Inuit? Evo and Proud, January 22. https://evoandproud.blogspot.com/2009/01/blond-inuit.html
Frost, P. (2010). Femmes claires, hommes foncés. Les racines oubliées du colorisme. Quebec City: Les Presses de l’Université Laval, 202 p. https://www.pulaval.com/livres/femmes-claires-hommes-fonces-les-racines-oubliees-du-colorisme
Frost, P. (2022). European Hair, Eye, and Skin Color: Solving the Puzzle. Washington: Academica Press. ISBN 9781680538724
Frost, P. (2025). Emotional responses to the differing skin tones of men and women. Peter Frost’s Newsletter, April 3.
Frost, P., Kleisner, K., & Flegr, J. (2017). Health status by gender, hair color, and eye color: Red-haired women are the most divergent. PLoS One, 12(12), e0190238. https://doi.org/10.1371/journal.pone.0190238
Günther, T., H. Malmström, E.M. Svensson, A. Omrak, F. Sánchez-Quinto, G.M. Kilinç, et al. (2018). Population genomics of Mesolithic Scandinavia: Investigating early postglacial migration routes and high-latitude adaptation. PLoS Biol, 16(1), e2003703. https://doi.org/10.1371/journal.pbio.2003703
Hoffecker, J.F. (2002). Desolate landscapes. Ice-age settlement in Eastern Europe. New Brunswick: Rutgers University Press.
Hollis, B., Day, F. R., Busch, A. S., Thompson, D. J., Soares, A. L. G., Timmers, P. R., ... & Wilson, C. H. (2020). Genomic analysis of male puberty timing highlights shared genetic basis with hair colour and lifespan. Nature communications, 11(1), 1536. https://doi.org/10.1038/s41467-020-14451-5
Hysi, P.G., Valdes, A.M., Liu, F., Furlotte, N.A., Evans, D.M., Bataille, V., et al. (2018). Genome-wide association meta-analysis of individuals of European ancestry identifies new loci explaining a substantial fraction of hair color variation and heritability. Nature Genetics, 50(5), 652-656. https://doi.org/10.1038/s41588-018-0100-5
Ikizler, A.S. (2007). Gender role representations in Turkish television programs, Submitted as a St. Mary’s Project in Partial Fulfillment of the Graduation Requirements, St. Mary’s College of Maryland for the Degree of Bachelor of Arts in Psychology http://citeseerx.ist.psu.edu/viewdoc/summary?doi=10.1.1.514.2218
Janif, Z.J., Brooks, R.C., & Dixson, B.J. (2015). Are preferences for women’s hair color frequency-dependent? Adaptive Human Behavior and Physiology, 1(1), 54-71. https://doi.org/10.1007/s40750-014-0008-y
Jones, E.R., Zarina, G., Moiseyev, V., Lightfoot, E., Nigst, P.R., Manica, A., et al. (2017). The Neolithic transition in the Baltic was not driven by admixture with early European farmers, Current Biology, 27(4), 576-582. https://doi.org/10.1016/j.cub.2016.12.060
Kažoka, D. & Vetra, J. (2011). Variations in some anthropometrical parameters of the women with the different iris color in Latvia. Papers on Anthropology, XX, 160-170. https://doi.org/10.12697/poa.2011.20.17
Kleisner, K., Kocnar, T., Rubešová, A., & Flegr, J. (2010). Eye color predicts but does not directly influence perceived dominance in men. Personality and Individual Differences, 49(1), 59-64. https://doi.org/10.1016/j.paid.2010.03.011
Kleisner, K., Priplatova, L., Frost, P., & Flegr, J. (2013). Trustworthy-looking face meets brown eyes. PLoS One, 8(1), e53285. https://doi.org/10.1371/journal.pone.0053285
Lazaridis, I., Patterson, N., Mittnik, A., Renaud, G., Mallick, S., Kirsanow, K., et al. (2014). Ancient human genomes suggest three ancestral populations for present-day Europeans. Nature, 513(7518), 409-413. https://doi.org/10.1038/nature13673
Lea-Greenwood, G. (1998). Visual merchandising: a neglected area in UK fashion marketing? International Journal of Retail & Distribution Management, 26(8), 324-329. https://doi.org/10.1108/09590559810231797
Lorenz, K. (1971). Studies in Animal and Human Behaviour, vol. 2. London: Methuen & Co.
Manning, J.T., Bundred, P.E., & Mather, F.M. (2004). Second to fourth digit ratio, sexual selection, and skin colour. Evolution and Human Behavior, 25(1), 38-50. https://doi.org/10.1016/s1090-5138(03)00082-5
Melchior, L., Lynnerup, N., Siegismund, H.R., Kivisild, T., & Dissing, J. (2010). Genetic diversity among ancient Nordic populations. PLoS One, 5(7), e11898 https://doi.org/10.1371/journal.pone.0011898
Mittnik, A., Wang, C-C., Pfrengle, S., Daubaras, M., Zarina, G., Hallgren, F., et al. (2018). The genetic prehistory of the Baltic Sea region. Nature Communications, 9(442) https://doi.org/10.1038/s41467-018-02825-9
Morgan, M.D., Pairo-Castineira, K. Rawlik, E., Canela-Xandri, O., Rees, J., Sims, D., Tenesa, A., & Jackson, I.J. (2018). Genome-wide study of hair colour in UK Biobank explains most of the SNP heritability. Nature Communications, 9, 5271. https://doi.org/10.1038/s41467-018-07691-z
Norton, H.L., & Hammer, M.F. (2007). Sequence variation in the pigmentation candidate gene SLC24A5 and evidence for independent evolution of light skin in European and East Asian populations. Program of the 77th Annual Meeting of the American Association of Physical Anthropologists, p. 179.
Olalde, I., Allentoft, M.E., Sanchez-Quinto, F., Santpere, G., Chiang, C.W.K., DeGiorgio, M., et al. (2014). Derived immune and ancestral pigmentation alleles in a 7,000-year-old Mesolithic European. Nature, 507(7491), 225-228. https://doi.org/10.1038/nature12960
Piffer, D. (2015). The origins and spread of blue eyes in Europe: Evidence from ancient DNA. PifferPilfer, September 19.
Posth, C., Yu, H., Ghalichi, A., Rougier, H., Crevecoeur, I., Huang, Y., ... & Krause, J. (2023). Palaeogenomics of upper palaeolithic to neolithic European hunter-gatherers. Nature, 615(7950), 117-126. https://doi.org/10.1038/s41586-023-05726-0
Schweder, B.I.M. (1994). The impact of the face on long-term human relationships. Homo, 45(1): 74-93.
Shekar, S.N., Duffy, D.L., Frudakis, T., Montgomery, G.W., James, M.R., Sturm, R.A., & Martin, N.G. (2008). Spectrophotometric methods for quantifying pigmentation in human hair-Influence of MC1R genotype and environment. Photochemistry and Photobiology, 84(3), 719-726. https://doi.org/10.1111/j.1751-1097.2007.00237.x
Simcoe, M., Valdes, A., Liu, F., Furlotte, N.A., Evans, D.M., Hemani, G., et al. (2021). Genome-wide association study in almost 195,000 individuals identifies 50 previously unidentified genetic loci for eye color. Science Advances, 7(11), eabd1239 https://doi.org/10.1126/sciadv.abd1239
Stansfield, J., & Whitfield, T.W.A. (2005) Can future colour trends be predicted on the basis of past colour trends? An empirical investigation. Color Research & Application, 30(3), 235-242. https://doi.org/10.1002/col.20110
Thelen, T.H. (1983). Minority type human mate preference. Social Biology, 30(2), 162-180. https://doi.org/10.1080/19485565.1983.9988531
van den Berghe, P.L., & Frost, P. (1986). Skin color preference, sexual dimorphism and sexual selection: A case of gene-culture co-evolution? Ethnic and Racial Studies, 9(1), 87-113. https://doi.org/10.1080/01419870.1986.9993516




>Thus, on the eve of recorded history, all Europeans now possessed a unique look that would later define them, as if they were a cast of actors being hastily made up and rushed onto stage moments before curtain time.
This is so tuff
The key environmental difference between NorthEast Asia and North-Mid Europe is the much stronger cloud coverage in Europe. Some have hypothesized that the small eye openings of modern-day Inuit and Tungusic groups are actually beneficial in the high-glare environments that they inhabit. Also, evidence suggests the east part of the steppe-tundra that started in Europe and went to above Mongolia was inhabited by ANE first, not by the phenotypically East Asian people that today inhabit the top part of eastern and central Siberia.
With that being said, the likely homeland of East Asians, the steppe tundra on the Pacific coast, could've been colder than the other steppe-tundra, as it is actually colder than the livable parts of Scandinavia today. East Asians also have a cold-adapted phenotype, but to a different type of environment than the ones Northern europeans adapted to.